Organisms Colonization Growth and Evolution in a Friendly Environment Review
Abstract
'Marine biofouling', the undesired growth of marine organisms such equally microorganisms, barnacles and seaweeds on submerged surfaces, is a global trouble for maritime industries, with both economical and environmental penalties. The primary strategy for combating marine fouling is to apply biocide-containing paints, but ecology concerns and legislation are driving science and technology towards non-biocidal solutions based solely on physico-chemical and materials properties of coatings. Advances in nanotechnology and polymer science, and the development of novel surface designs 'bioinspired' by nature, are expected to have a significant affect on the development of a new generation of environmentally friendly marine coatings.
Introduction
'arine biofouling, the colonization of submerged surfaces past unwanted marine organisms (Fig. 1), has detrimental effects on shipping and leisure vessels, oestrus exchangers, oceanographic sensors and aquaculture systems. For instance, information technology has been shown that the increased roughness presented by a heavily fouled ship hull can event in powering penalties of upward to 86% at cruising speed; even relatively light fouling by diatom 'slimes' tin can generate a 10–sixteen% penalisation1. Without constructive antifouling (AF) measures, in lodge to maintain speed, fuel consumption (and therefore greenhouse gas emissions2) increase significantly. A recent analysis of the economical impact of biofouling for the Arleigh Burke DDG-51 destroyers, which comprise 30% of the ships in the U.s. Navy armada, estimates the overall cost associated with hull fouling at $56 1000000 per annum3. This effigy is based on the present AF coating arrangement, cleaning and fouling level (typically heavy slime) of the Navy. If the assay is extended to the unabridged US Navy fleet, the gauge cost of hull fouling is between $180 and 260 million per annum.
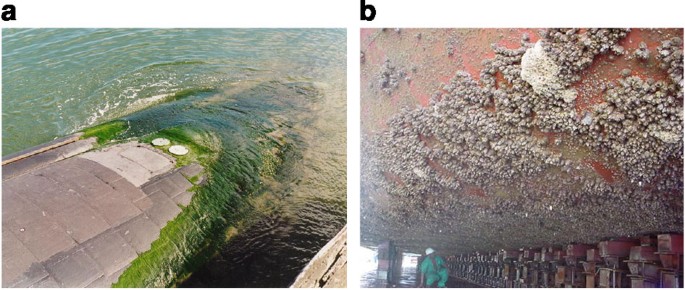
Images show (a) fouling past the green alga (seaweed) Ulva (epitome courtesy of Dr J. Lewis) and (b) barnacles (paradigm courtesy of Dr C.D. Anderson).
Marine biofouling is ubiquitous and has been a applied problem ever since human sailed the oceans; decision-making it, without simultaneously creating unacceptable environmental impacts on not-target species is a considerable challenge. Recent years have seen a resurgence of interest in the fundamental science behind the processes involved in biofouling, and in the design of novel coatings and other non-coating technologies. The master driver for this is legislation that has outlawed some highly constructive AF paints, notably the use of tributyltin oxide, and posed a stricter evaluation and regulatory authorities on the use of alternative biocides. 'Green' alternatives to biocide-based technologies are therefore urgently sought by the marine coatings manufacture, and there is considerable interest in developing biocide-free coatings that rely on surface physico-chemical and bulk materials backdrop to either deter organisms from attaching in the first place ('prevention is better than cure') or reduce the adhesion strength of those that do adhere, so that they are hands removed past the shear forces generated past ship movement or balmy mechanical cleaning devices.
Fouling-resistant coatings for application to large-scale structures, such as send hulls, are based on polymeric 'binders'—the picture-forming component of paint. Advances in macromolecular synthesis, self-assembling polymers, hyperbranched and interpenetrating polymer networks and various types of nanocomposites, provide opportunities for developing novel folder systems. Natural AF surfaces also provide a source of inspiration for new coating designs.
This review is aimed at the intersection between biological science, materials sciences and engineering. Biological aspects of fouling and the particular challenges posed to the materials scientist confronted by the vast biodiversity in fouling species and the range of attachment behaviours and adhesion mechanisms adopted will kickoff be considered. Some of the novel approaches currently being explored by materials scientists in developing coatings for marine applications, both from a primal and more practical perspective, volition then exist discussed. Emphasis volition be given to interdisciplinary studies in which the structure and surface properties of coatings are correlated with their biological performance or in which predictive models are being developed that will facilitate rational blueprint of coatings in the future.
Fouling organisms and their settlement and adhesion strategies
When a clean surface is immersed in natural seawater, it immediately starts to adsorb a molecular 'workout' film primarily consisting of dissolved organic fabriciv. Colonization of the conditioned surface by a wide range of organisms depends on the availability of the colonizing stages, and their relative rates of attachment and surface exploitation. Fouling is a highly dynamic process; the specific organisms that develop in a fouling customs depend on the substratum, geographical location, the season and factors such as contest and predation.
A stardom is often made among 'microfouling' (often referred to as 'slime') due to unicellular microorganisms such equally bacteria, diatoms and protozoa, which course a complex biofilm; 'soft macrofouling' comprising macroscopically visible algae (seaweeds) and invertebrates such equally soft corals, sponges, anemones, tunicates and hydroids; and 'hard macrofouling' from shelled invertebrates such every bit barnacles, mussels and tubeworms (Fig. 2).

(a) Bacteria (scanning electron micrograph (SEM)), (b) false-colour SEM of motile, quadriflagellate spores of the light-green alga (seaweed) Ulva, (c) faux-colour ecology SEM image of settled spore of Ulva showing secreted annulus of swollen adhesive, (d) SEM of diatom (Navicula), (e) larva of tube worm, Hydroides elegans (paradigm courtesy of B. Nedved), (f) barnacle cypris larva (Amphibalanus amphitrite) exploring a surface past its paired antennules (image courtesy of N. Aldred), (g) adult barnacles (epitome courtesy of As Clare), (h) developed tubeworms (H. elegans; image courtesy of M. Hadfield), (i) adult mussels showing byssus threads attached to a surface (paradigm courtesy of J. Wilker), (j) private plants of the green alga (seaweed) Ulva. The diagram is intended to indicate relative scales rather than absolute sizes; individual species within a group tin vary significantly in absolute size.
It is often stated that surface colonization follows a linear 'successional' model5,vi,7,8,nine in which bacterial biofilm formation is followed inside a week by spores of macroalgae (seaweeds), fungi and protozoa, followed in plough, within several weeks, by larvae of invertebrates, such every bit barnacles. In reality, this 'classical' view is a considerable oversimplification equally motile spores of seaweeds are capable of settling within minutes of presenting a clean surfacex and larvae of some species of barnacles, bryozoans and hydroids settle inside a few hours of immersionxi. For both algae and larvae, this is well inside the 'ane-to-several weeks' quoted past Wahl6. Rather than the linear successional model, the 'dynamic' model12,13 provides a more counterbalanced view.
Information technology is also misleading to assume that successional colonization of a surface necessarily implies a causal relationship between 1 phase and the adjacent and fifty-fifty more misleading to assume that decision-making or blocking initial stages of colonization, such as biofilm formation, will reduce or eliminate macrofouling. On the other hand, information technology is undoubtedly articulate that attachment of spores and larvae tin be influenced past other organisms, notably by bacterial biofilms, and positive, negative and neutral effects have been detected in controlled laboratory experiments when biofilms of specific bacteria accept been tested against algal spores and larvae of invertebrates14,15,16,17,18. Some of these effects take also been reported in field observationsxvi. However, colonization models are mostly based on studies of natural substrata, such as wood and stone or traditional AF coatings that rapidly foul, and there are only a few reports that study the colonization of modern, not-biocidal AF coatings by microorganismsnineteen,20. Fouling by biofilms composed of communities of bacteria, diatoms, fungi and protozoa has been largely ignored until recently, because the focus of inquiry has been on macrofouling. It is probable that more attention will exist paid to slime fouling in future, at present that its economical impact is firmly established1,3.
A major challenge in creating an effective fouling-resistant coating is that the variety of fouling organisms is vast and the range of adhesion mechanisms (including adhesives) used is correspondingly great (Fig. 2). The colonizing ('recruitment') stages of fouling organisms range in size from micrometres (bacteria, unmarried-celled spores of algae and some diatom cells) to hundreds of micrometres or fifty-fifty millimetres (larvae of invertebrates). Considerations of size are relevant to attempts (encounter Futurity Directions) to engineer surface topographies that may deter the settlement of organisms. Nonetheless, the critical length scale in determining settlement is not necessarily the size of the organism per se, but rather the size of the parts or structures involved in the sensing appliance of an organism (for example, the paired sensory antennules of barnacle cypris larvae21 (Fig. two)), which decide whether the organism selects a surface for zipper.
Assuming that the organism has settled, the success of that organism in colonizing a surface and growing into a reproductive adult, within a turbulent marine environment, depends on how well the macromolecular adhesive polymers secreted by the settled organisms secure to the agglutinative interface, which is adamant by the interfacial molecular interactions that are in plough influenced by the properties of a surface or coating at the molecular or nanoscale level. Not-biocidal, fouling-resistant coatings are based on polymers designed to minimize molecular adhesive forces between the adhesives used by marine organisms and the coating. Rational pattern of coatings ultimately requires an elucidation of the chemic and physical interactions betwixt the bioadhesives used past marine organisms and the prospective fouling-release polymer(south). Unfortunately, so few marine bioadhesives have been isolated and characterized that obtaining information at this level has proved to be difficult (run into ref. 22 for comprehensive reviews of bioadhesives). An exception to this is the well-characterized marine mussel foot adhesive system based on byssal threads and agglutinative plaques formed from a composite of several proteins23. Catechol groups of the amino acid DOPA (dihydroxyphenylalanine) are oxidized to react with other catechol groups to crosslink the proteins into a potent cohesive composite. Dihydroxyphenylalanine groups also hydrogen bail to metal ions in the mineral substrata. Surface-sensitive spectroscopic techniques have been used to sympathise molecular interactions betwixt mussel adhesive proteins and diverse interfaces in situ. For case, sum frequency generation vibrational spectroscopy (SFG) has shown how substrate surface chemical science influences the structure of the adsorbed adhesive plaque protein Mefp-three24. On a hydrophobic, non-polar substrate, to which Mefp-three attaches weakly, strong SFG signals were detected in both C–H stretching and amide I regions, indicating that the adsorbed protein was in an ordered conformation. On a polar, hydrophilic polymer substrate to which Mefp-3 strongly adheres, no SFG signals in these regions were detected, showing that the poly peptide adopted a more than or less random or matted structure. Application of this type of analysis to films based on polymers used in fouling-release coatings will evidence instructive; all the same, until more marine adhesives of important fouling organisms are biochemically characterized and fabricated available in purified form and in sufficient quantity for experimental purposes, the biophysical assay of molecular events at agglutinative/coating interfaces will have only limited touch on on the pattern of novel coatings.
Insights from in situ imaging of surface exploration
One approach to the development of novel AF coatings is to create a 'deterrent' surface that inhibits the initial attachment of the recruitment ('settling') stages of spores, larvae and so on. These settling stages are 'choosy' in the sense that they showroom behaviours that consequence in a surface being accepted or rejected for settlement. Although much can exist done to assess such behavioural traits through simple assays of the ability of larvae or spores to settle on a surface, a more comprehensive understanding requires detailed observation of the behaviour of organisms equally they explore the interface. In this section, nosotros outline two electric current imaging technologies that are being used to raise our understanding of organism behaviour in relation to specific surface properties. This information will provide improved insight into the chemistry and thermodynamics of adhesion and will assistance to codify the 'design rules' by which novel materials may be developed to deter settlement.
Two-dimensional tracking of the cypris larvae of barnacles has been studied using EthoVision tracking software25. Aldred et al.26 explored cyprid behaviour on two zwitterionic polymer coatings (poly(sulfobetaine methacrylate) (polySBMA) and poly(carboxybetaine methacrylate) (polyCBMA)), both of which completely inhibited cyprid settlement and are resistant to protein adsorption27. On polySBMA and on bare glass, cyprids explored the surface, but were unwilling or unable to settle (Fig. three), whereas on polyCBMA, cyprids did non endeavor exploration and left the surface quickly.
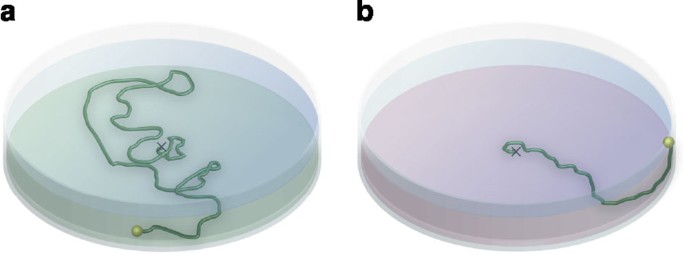
The motile cypris larvae of barnacles are 'choosy', that is, they explore the suitability of a surface for eventual adhesion by swimming over it, making 'sampling' contacts. The cartoon illustrates an experiment in which the bottom surface of 2 transparent dishes was coated with either (a) poly(sulfobetainemethacrylate) (polySBMA) or (b) poly(carboxybetainemethacrylate) (polyCBMA). (Note: The two coatings are actually colourless, but are shown as different colours to help understanding). The dishes were filled with seawater before introducing the barnacle cypris larvae. The dark-green lines illustrate the representative tracks of an individual larva on each surface, starting at or near the middle of the dish. Behaviour on polySBMA (a) is characterized by broad, sweeping deviations in the recorded rail, with occasional contacts with the surface and abrupt changes in direction. This behavior suggests that the larvae are actively exploring this surface. Behaviour on polyCBMA (b) is quite different. Here, the larvae spent little fourth dimension either swimming over the surface or making contacts with it, swimming immediately to the border of the dish. Cartoon represents original information in ref. 26.
Although tracking the behaviour of fouling organisms aids in agreement surface pick, a more than detailed understanding of interfacial interactions also requires information at the molecular level. Imaging surface plasmon resonance (iSPR) has been practical to in situ, real-time analysis of temporary adhesive deposition by barnacle larvae as they explore a surface28. Footprints of temporary adhesive were imaged on a bare gold-coated surface and a self-assembled monolayer of oliogoethylene glycol, selected for its well-known resistance to protein adsorption. Larvae were observed to probe both surfaces with their sensory structures, just merely on the blank gold surface did the betoken of contacts leave deposits of temporary adhesive (Fig. four). The frequency with which the secreted poly peptide sticks to the surface provides a proxy indication of the forcefulness of the protein-surface bail28. This example demonstrates the ability of iSPR to generate novel information on the exploration of different surfaces at the molecular level; what is now needed is to apply this approach to more than circuitous, candidate experimental coatings for marine application, rather than simple model substrates, such as OEGs (oligo(ethyleneglycol))—this may require further technical improvements to iSPR.
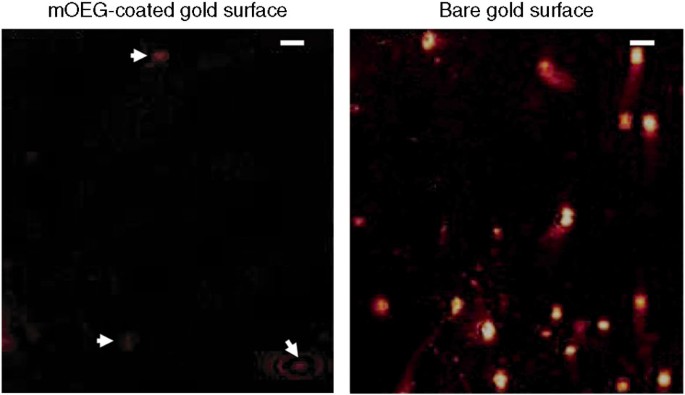
SPR is a spectroscopic, surface analysis technique that allows the adsorption of macromolecules to a metallic surface to be measured and imaged through changes in reflectivity. The ii panels show snapshots of aureate surfaces later cypris larvae were allowed to explore for 7 min. Ane of the gilt surfaces was coated with methyl-terminated oligo(ethyleneglycol) (mOEG), the other was left every bit bare gilded. On the blank gold surface, the cyprids explored the surface via their sensory antennules and made numerous contact points or 'footprints', shown every bit bright cogitating spots, each of which represents the deposition of small-scale amounts of proteinaceous temporary adhesive from the antennules. On the golden surface, these spots persisted post-obit detachment of the cyprid, implying that the adhesive remained on the surface. In the instance of the mOEG substrate, which is well known for its poly peptide-repellent properties, although the cyprids explored the surface, very little proteinaceous residue remains at the contact points, as shown by the few, dull-cogitating spots (arrowed). On mOEG, the spots only persisted for as long as the cyprid remained in contact with the surface implying that, on detachment, the protein remained on the antennules. The calibration bars in each frame are 100 μm. Reproduced with permission from ref. 28, © 2008 American Vacuum Society.
Novel non-biocidal coating strategies
Two general (non-exclusive) strategies are typically followed in the design of novel, non-biocidal, non-fouling surfaces. In this review, nosotros distinguish between AF coatings, in which the objective is to deter the recruitment stages of fouling organisms from attaching in the first place, and 'fouling-release' (FR) coatings, which do not prevent organisms from attaching, simply the interfacial bond is weakened so that attached organisms are more easily removed by the hydrodynamic shear forces generated by move of the ship through the h2o29 or by gentle 'grooming' devices30. In both cases the objective is to achieve the desired result through manipulation of the physico-chemical and/or materials properties of the coating (for example, rubberband modulus, frictional coefficient) so that the organism either perceives the surface as unconducive to settlement or the intermolecular interaction forces between the surface and the polymeric adhesives produced by the fouling organism are weakened, promoting agglutinative failure. These two general approaches are not mutually sectional and in fact the distinction is overly simplistic.
Most current commercial FR coatings are based on poly(dimethylsiloxane) elastomers (PDMSe)31. These non-polar, low surface free energy (∼22 mN m−one), hydrophobic polymers would exist expected to prove low adhesion of polar molecules (including agglutinative proteins) because of reduced opportunities for H-binding and polar interactions. Coatings based on PDMSe also have typically low elastic moduli, and the release of hard-fouling is proportional to (γE)½; where γ is the surface energy and E is the modulus32. Although PDMSe showroom the desired combination of low surface energy (to minimize the work of adhesion) and low modulus, such coatings endure from some disadvantages. Because of their low surface energy, they are hard to bond to a substrate without an advisable necktie coat. They are less durable, more easily damaged than other types of blanket and they frequently 'fail' to dark-brown slimes dominated by diatoms that adhere more strongly to hydrophobic surfaces19,33. The fouling-release technology is too most effective when applied to high-activity, fast-moving (>fifteen knot) vessels and is less suitable for vessels that spend long periods in port or which cruise at lower speeds to maintain fuel efficiency. For these and other reasons, there has been extensive inquiry on newer fouling-release technologies31, which may either 'toughen up' silicones through the use of new tie-coats and the incorporation of fluoropolymers or which seek to create a surface complexity. Currently in faddy are amphiphilic coatings, which comprise some of the benefits of both hydrophobic and hydrophilic functionalities, for example, the latest fouling-release blanket from International Paint, Intersleek 900. Such coatings, through phase separation, for instance, of mutually incompatible block copolymers, create a dynamic surface with local variations in surface chemistry, topography and mechanical properties. The general aim is to create a dynamic and compositional surface complication that deters settlement stages of fouling organisms and reduces the interfacial bonding with adhesive polymers.
Here we review the range of experimental blanket technologies currently being explored as the footing for future practical AF coatings. Merely a few of these technologies have reached the stage of evaluation in the field, for instance, as test panels on rafts, and there are no published reports of field performance. The master focus will exist on polymer-based experimental systems that can be envisaged every bit the basis for futurity practical coatings for application to ship hulls. The accent volition be on studies that demonstrate clear coating structure/property/functioning correlations and the importance of using surface characterization techniques nether in situ (that is, immersed) weather in order to assess surface reorganization and to understand the type of surface that the settling stages (cells and larvae) meet. The section will too consider the current involvement in biomimetic/bioinspired technologies and efforts to rationalize the influence of surface roughness through predictive models.
Bioinspired engineered topographies
Information technology is often observed that many marine organisms do not become colonized by other specieseight,34,35,36,37. A diverse range of mechanisms has been implicated in natural defense, including settlement-inhibiting micro- and nanotopographies, secreted bioactive molecules, sloughing surface layers, mucus secretions and hydrolytic enzymes (run across ref. 38 for review). Natural mechanisms may be used as the footing for 'biomimetic' or 'bioinspired' coatings, and virtually attending has been devoted to designs based on topographical features. The surfaces of many marine animals ranging from shells of molluscs to the skin of sharks and whales have a circuitous surface topography, and past illustration with the 'self-cleaning' lotus-leaf effect, it is often speculated that this surface roughness may take a role in either deterring fouling organisms from attaching or promoting their easy release. These thoughts accept encouraged research on a number of bioinspired surface designs35 of which the most prominent for marine applications are those that mimic sharkskin39 and invertebrate shellstwoscore.
Moulded topographies in PDMSe (Box ane), inspired by the pare of fast-moving sharks at ∼ane/25th of the scale (Sharklet AF, Fig. 5), resulted in an 85% reduction in settlement of zoospores (motile spores) of the macroalga Ulva compared with smooth PDMSe39. The Sharklet AF topography consists of 2μm wide rectangular-like (ribs) periodic features (4, eight, 12 and 16 μm in length) spaced 2 μm apart. Farther studies based on 2μm features correlated zoospore settlement with an empirically derived 'engineered roughness index' (ERII) for various surface designs41,42. ERII is a dimensionless ratio involving several geometric parameters of the surfaces:

where r is the Wenzel's roughness factor, (i−ϕ southward ) is the surface area fraction of feature tops (that is, the ratio of the depressed surface area between features and the projected planar surface area) and df is the degree of freedom of spore movement (one or 2). Spore settlement decreased with an increase in ERII. Sharklet AF with an ERII of 9.five showing a 77% reduction in settlement compared with the smooth surface.
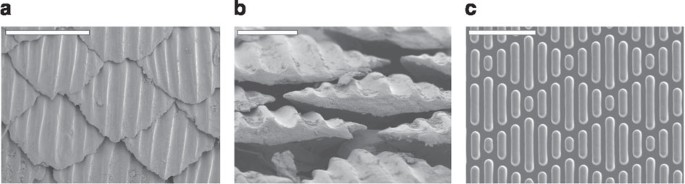
The scanning electron micrographs show the skin denticles of spinner shark in face (a) and terminate (b) views and (c) prototype of Sharklet AF topography moulded in PDMSe. Scale bars are (a) 500 μm, (b) 250 μm and (c) 20 μm. Images courtesy of: Professor A.B. Brennan.
Schumacher et al.43 introduced the concept of nanoforce gradients to explain how dissimilar feature geometries based on the Sharklet design might exert an influence on the settlement of spores. It was hypothesized that nanoforce gradients caused by variations in topographical feature geometry will induce stress gradients within the lateral plane of the cell membrane of a settling jail cell during initial contact. Perception of such stress gradients by presumed mechanotransducer proteins in the cell membrane could, through intracellular signal cascades, lead to modification of the settlement response. The generation of the nanoforce gradients was envisaged as a office of the angle moment or stiffness of the topographical features with which the prison cell is in contact. The geometric dimensions, including width, length and height of the topographical feature, as well as the modulus of the base fabric, define its stiffness. By introducing geometric variations in features independent in the engineered topography, an effective force gradient between neighbouring topographical features is developed. To examination the nanoforce gradient hypothesis, modifications were made to the design of Sharklet AF, resulting in a range of nanoforce gradients43. The surfaces were then challenged with spores of Ulva. Surfaces with nanoforce gradients ranging from 125 to 374 nN all significantly reduced spore settlement relative to a smooth substrate, but the level of inhibition did non correlate directly with the magnitude of the slope, nor was the level of inhibition predicted by ERII. It became evident that the number of singled-out features was a improve predictor of inhibition by the topographies and this led to exchange of df past due north, in a revised ERI model (ERITwo).

Settlement densities of spores of Ulva were affected past the complexity of the microtopographical patterns, settlement decreasing equally the number of features increased (Fig. 6). The number of attached spores per unit area was normalized to the number of spores attached to a smooth control and the data were transformed past taking the natural logarithm (ln A/A o). The spore settlement density on the unlike patterns correlated well with the number of features and the ERI (ERIIi) model42. Furthermore, the level of spore settlement on several novel surface designs and some designs tested previously was correctly predicted by the ERITwo model42.
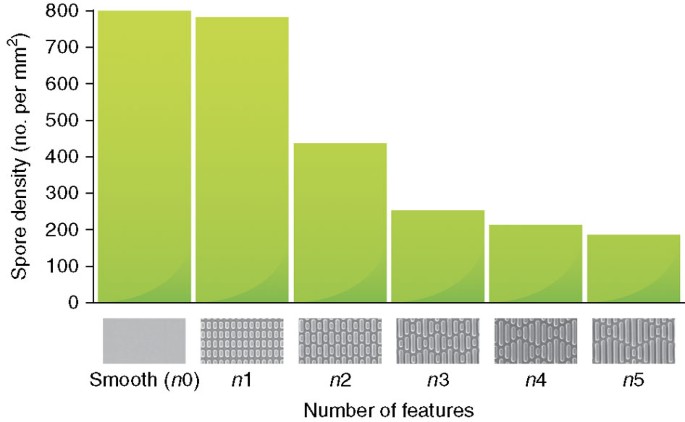
The graph shows the results of an experiment in which spores of Ulva were allowed to settle (adhere) to a variety of 'Sharklet-type' patterns, with increasing numbers of distinct topographic features. The height of the bars indicates the resulting density of spores settled on the different patterns, which are illustrated below each bar (from left to right, n=0 (smooth), i–v, where n=the number of distinct topographic features). In all cases, the height and spacing of the features remained constant (2.eight μm high×two μm broad×two μm infinite). Figure drawn from data in ref. 42; images of patterns courtesy of Professor A.B. Brennan.
These results confirm that the designed nanoforce gradients may be an effective tool and predictive model for the design of unique non-toxic, non-fouling surfaces for marine applications. Even so, as dissimilar fouling organisms respond to topographies of unlike length scales, hierarchical patterning may be required. An initial written report by Schumacher et al.44 indicated that complex designs would be required to repel multiple settling species. Electric current research on the 'Sharklet effect' attempts to combine topography with different chemistries at the surface or in the bulk, to further intensify the deterrent effect. It should also be pointed out that to exist successful, bioinspired technologies volition require multiple attributes (topography, modulus and chemistry) to be effective in the marine environment. The organisms that have provided the inspiration for this area of inquiry, for example, mussels, utilise several strategies to go on themselves 'clean'34, and hierarchical topographic designs will be needed to combat settlement of all cells and larvae44.
Amphiphilic nanostructured coatings
A contempo trend in designing experimental coatings for AF/fouling-release purposes has been to create surfaces with compositional (chemical) heterogeneity at the nanoscale through the thermodynamically driven, stage segregation of polymer assemblies, followed by crosslinking in situ. These coating designs may exist based on blends of immiscible polymers or contrasting chemistries of block copolymers, and the general aim is to combine the not-polar, low surface energy properties of a hydrophobic (typically fluorinated) component to reduce polar and hydrogen-bonding interactions with the bioadhesives used by fouling organisms, with the well-known poly peptide repellency backdrop of the hydrophilic components, typically, oligo(poly)ethylene glycols. The resulting chemical ambiguity, expressed in terms of amphiphilic nanodomains on the surface (Fig. 7), may lower the entropic and enthalpic driving forces for the adsorption of the marine protein and glycoprotein bioadhesives, which are themselves amphiphilic in character45,46. Lau et al.47 have speculated that the ofttimes-observed protein resistance of some nanopatterned, amphiphilic diblock copolymers is due to the intrinsic high density of surface interfacial boundaries.
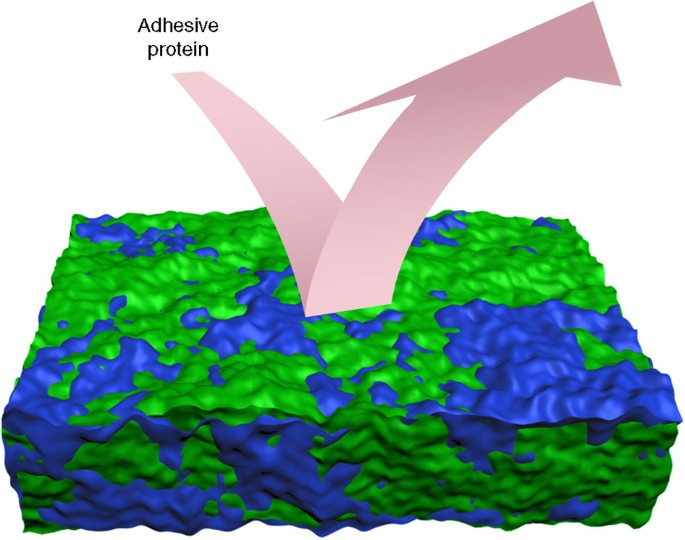
Such chemically 'ambiguous' coatings could be based, for example, on the combination of fluorinated, hydrophobic segments (greenish) and hydrophilic poly(ethyleneglycol) segments (blue), thus imparting an amphiphilic grapheme. The relative scale of the amphiphilic patches is non intended to indicate accurate dimensions. Cartoon of amphiphilic surface courtesy of Professor Karen Wooley.
The kickoff published example of an amphiphilic coating for employ in marine AF is that of ref. 48, based on hyperbranched fluoropolymers and linear poly(ethylene glycols) (PEG), which self-assemble on cantankerous-linking to form complex surface topographies and chemical domains of both nanoscopic and microscopic dimensions. The surface patterns were strongly influenced by immersion and by the relative proportions of the 2 polymers. The coating design anticipated that this surface complexity would either have a deterrent effect on the settling stages of fouling organisms or would be unfavourable for adsorption and unfolding of adhesive proteins. Gudipati et al.49 subsequently showed that several macromolecules of biological origin (proteins and lipopolysaccharides) showed reduced adsorption to compositions with loftier concentrations of PEG (45–55% past weight). These compositions were too effective confronting settlement of zoospores of the greenish alga Ulva, and minor plants of Ulva adhered less well to some of the compositions compared with PDMSe.
Krishnan et al.50 used side-concatenation grafting of hydrophilic oligoethylene glycol and hydrophobic perfluoroalkyl side chains to a preformed poly(styrene-cake-acrylic acid) copolymer. Martinelli et al.51,52 developed similar amphiphilic copolymers using controlled atom transfer radical polymerization and a purely polystyrene backbone. In both cases, these surface-active block copolymers were deposited equally a sparse moving-picture show over a thick layer of SEBS (poly(styrene-block-ethylene-random-butylene)-block-polystyrene), an elastomeric thermoplastic cloth that provides a suitable low modulus53. In both studies, NEXAFS and angle-resolved 10-ray photoelectron spectroscopy measurements demonstrated that the film surface underwent reconstruction resulting from the thermodynamically induced phase segregation of the mutually incompatible components. However, these are in vacuo techniques and it is difficult to translate the data they provide on surface structure in the dry state to the situation when these coatings are immersed in h2o. To empathise the human relationship between surface properties and biological operation, information technology is necessary to perform surface characterization in the immersed state. In situ (underwater) atomic force microscopy showed that surface topography changed on immersion, presumably acquired by localized swelling of the hydrophilic blocks51 (Fig. 8). Information on the surface chemistry of immersed coatings was provided by Sum Frequency Generation spectroscopy, which showed that, in water, the hydrophilic PEG segments migrated to the surface and surprisingly the hydrophobic, fluorinated groups were withal axiomatic, suggesting that the surface is truly amphiphilic in nature54. These coatings have been tested in bioassays to explore their intrinsic ability to resist the settlement and reduce the adhesion strength of two marine algae, namely, the macroalga (seaweed) Ulva and the unicellular diatom Nafiveicula. These organisms were called specifically considering they show contrary preferences, Ulva tends to adhere most strongly to hydrophilic coatings while diatom species such as Navicula attach most strongly to hydrophobic coatings, particularly those that are silicone-based. Both Ulva and Navicula showed weak adhesion to the amphiphilic surface-active block copolymers coatings compared with a standard fouling-release coating of PDMSe46,51.
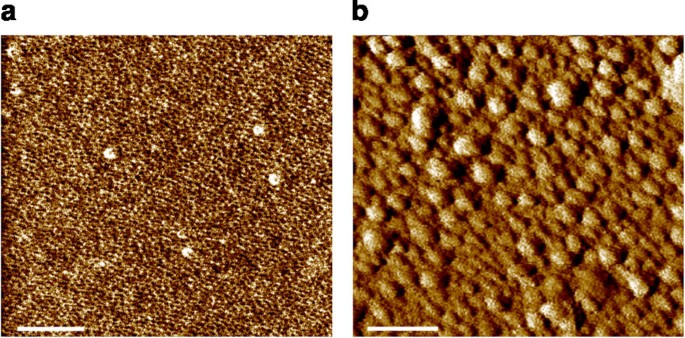
AFM phase images of polystyrene-based amphiphilic diblock fluorinated/PEGylated copolymer coatings were obtained in tapping mode (a) before, and (b) after immersion for 7 days in artificial seawater. Scale bars are 200 nm. Images reproduced with permission from ref. 51, © 2008 American Chemic Society.
Phase-segregating siloxane–polyurethane copolymers
In an attempt to improve the mechanical backdrop of PDMS elastomers, thermosetting, cantankerous-linked PDMSe–polyurethane nanohybrid copolymer coatings have been synthesized55,56,57,58. Sure compositions (those containing just 10% PDMSe) spontaneously phase-separated to form microtopographic domains equanimous of PDMSe surrounded by the polyurethane matrix. The domains appeared to increase in size after two-week immersion: stability depended on the casting solvent55 and mixing time57 used. Compositions with a higher proportion of PDMSe did not form domains, simply rather formed a polish stratified coating of depression surface energy PDMSe over a sub-layer of higher surface energy polyurethane. In detachment studies with 'pseudobarnacles' (an epoxy-bonded stud used as a proxy for live barnacles) and with reattached alive barnacles, it was shown that the pull-off force in both cases was lower on compositions with surface domains than those without57.
Majumdar et al.59 investigated the AF and fouling-release backdrop of PDMSe with tethered fourth ammonium salts (QAS). The initial concept was to combine the biocidal backdrop of QAS with the fouling-release properties of PDMSe. Surface assay of the resultant coatings revealed a heterogeneous, 2-phase morphology with increased water contact angle (increased hydrophobicity). Depending on the QAS alkyl chain length, surface protrusions were either isolated, 0.7–2.6 μm in size (C14), or inter-connected, 0.lx–two.9 μm in size (C18). The C18 coatings as well showed college levels of nanoroughness. In laboratory tests with a range of fouling organisms, the rougher C18 coatings showed the all-time fouling-release performance confronting the macroalga Ulva exceeding that of a commercially available FR coating. FR performance against Ulva is unlikely to be related to the tethered biocidal functionality per se, equally the coatings did not affect growth of the alga, but rather seems to exist related to the nanoroughness. On the other mitt, it was shown that the QAS coatings inhibited microbial biofilm germination of the bacterium Cellulophaga lytica and the diatom Navicula incerta—which is most probable due to the biocidal functionality.
Superhydrophilic zwitterionic polymers
The limitations in terms of stability of protein-resistant molecules such as PEG for marine applications accept been referred to earlier. A more promising group of chemistries that deter the adsorption of proteins and cells are zwitterionic materials, such as poly(sulphobetaine) and poly(carboxybetaine). These materials accept good chemic stability and low costthreescore. The majority of the published papers on zwitterionic coatings chronicle to proteins, serum or medically important bacteria (reviewed in ref. 60). In AF assays polySBMA brushes grafted onto glass surfaces through surface-initiated cantlet transfer radical polymerization almost completely inhibited the settlement of spores of the light-green alga Ulva and the attachment of diatom cells was also strongly reduced. In both cases, the few cells that did settle were only loosely attached61. Both polySBMA and polyCBMA resisted settlement of barnacle cypris larvae, just equally noted in a higher place (Insights from in situ imaging of surface exploration), larval searching behaviour differed on the two zwitterionic surfaces26. The resistance of zwitterionic materials to the adsorption of proteins and cells is generally attributed to a stiff electrostatically induced hydration layer that creates a superhydrophilic surface. In the case of marine organisms this means that the secreted proteoglycan bioadhesives are unable to accomplish a strong interfacial bond by excluding water molecules from the interface. The time to come evolution of hydrolyzable zwitterionic esters as coatings should provide a platform for the development of applied marine coatings60.
Inorganic–organic nanohybrids
Organically modified, hybrid xerogel coatings prepared by the sol-gel process have been shown to possess AF and fouling-release characteristics62,63,64,65. The xerogel surfaces are cheap and robust, characterized past uniform surface roughness/topography and cover a range of wettabilities and surface energies. The settlement of barnacle cyprids and algal zoospores was highly correlated with surface energy and wettability, equally was the removal of algal (Ulva) sporelings (immature plants) and adhered diatoms62,65. Withal, the response to these surface parameters was non uniform; cypris larvae of Balanus amphitrite prefered to settle on xerogel surfaces with high wettability and high surface energy65, while zoospores of Ulva prefered to settle on hydrophobic xerogel surfaces with depression surface energy62. Sporelings of Ulva were more readily removed from surfaces with low wettability and low surface energy (Bennett et al.62), while diatoms were more than readily removed from surfaces with loftier wettability and high surface energy65. The electric current xerogel formulations are only suitable for low-fouling freshwater environments in which slime predominates, as the thin (∼ane μm) xerogels do not take fouling-release backdrop for adult barnacles. To improve AF functioning in marine environments, sequestration of catalysts, for case, diorganoselenoxides and diorganotellurides, into the xerogel films that facilitate the oxidation of halide salts with naturally occurring hydrogen peroxide to course the corresponding hypochlorous acid have shown hope confronting the settlement of barnacle and tubeworm larvae and algal spores64.
Nanocomposites and superhydrophic surfaces
Hydrosilation-cured silicone elastomers, reinforced through the incorporation of pocket-size quantities of multiwall carbon nanotubes (MWCNTs) and sepiolite nanoclays, showed enhanced fouling-release performance in laboratory assays with algae and barnacles compared with unfilled PDMSe controls66. The utilize of MWCNTs is especially pregnant, as the improved performance was obtained at low loadings of the nanofiller (0.05–0.two% by weight). At this level of loading the bulk properties of the coatings (tensile modulus and cross-link density) announced to be unchanged, merely the coatings became slightly more hydrophobic and at that place was a meaning change in shear-thinning behaviour67. The changed rheological properties were attributed, on both theoretical and experimental grounds, to a strong molecular affinity between the siloxane chains and the MWCNTs, through CH-π interactions involving methyl groups of the PDMSe and effluvious rings of the MWCNTs67. This affinity is also of import in reducing the likelihood of MWCNTs being released into the marine (or any other) environs, which is relevant to the current contend on potential issues of nanoparticle toxicity. The improved fouling-release performance appears to exist correlated with the effect of MWCNTs in inducing fourth dimension- and immersion-dependent changes in surface nanotopography68. Nether tapping-mode AFM, both unfilled and MWCNT-filled PDMSe showed a shine morphology (root mean squared (RMS) roughness ∼1 nm), but after immersion in h2o, at that place was a complex fourth dimension-dependent surface restructuring of both filled and unfilled coatings, and those containing 0.one% MWCNTs exhibited a significant restructuring at the nanoscale. Information technology is non yet clear how such nanostructuring contributes towards fouling-release performance.
Superhydrophobic surfaces are water-repellent, having a high water contact angle (typically >150°) and a very low whorl-off angle, that is, the inclination angle at which a water driblet rolls off the surfaces69. The superhydrophobic outcome relies on the trapping of air, as exemplified by a number of natural surfaces, for example, the lotus foliage and insect wings37. Recently, Scardino et al.36 reported three superhydrophobic coatings (SHC) that inhibit the settlement/zipper of spores of algae and larvae of several invertebrates. The coatings were made by spraying fumed silica-filled siloxanes. All three coatings were superhydrophobic (SHC ane contact angle θ A =169°, SHC 2 θ A =155° and SHC three θ A =169°) and exhibited either micro/nanoroughness (SHC 1 and ii) or only nanoroughness (SHC 3). All exam organisms avoided settling on the SHC 3 blanket, which had large pores of ∼350 nm in diameter and pocket-size pores in the range of 10–50 nm. Minor-angle X-ray scattering was used to characterize the partial wetting of the superhydrophobic surfaces in situ under immersion conditions. The broad-spectrum AF effect was attributed to the larger amount of unwetted interface on SHC 3 compared with SHC 1 and 2 when immersed in seawater. Whether the unwetted interface will resist wetting (and hence the aggregating of fouling) following long-term immersion needs to be determined.
Future directions
Current commercial, non-biocidal fouling-release coatings, suffer from a number of drawbacks and correspond just a modest proportion of the total marine coatings market31. In the nearly future it is likely that coatings to combat marine biofouling will exist based on what are considered to be 'eco-friendly' biocides (for example, by tethering to the coating or by using biocides with low toxicity and curt half-lives)70. Yet, we take shown in this review that significant enquiry efforts are being directed towards the discovery or improvement of novel, not-toxic, non-fouling coatings equally alternatives to those currently used commercially. Nosotros have given several examples demonstrating that fouling organisms are influenced in their initial surface colonization behaviour, and the subsequent development of adhesion strength, by nano- and micro-scale-engineered coating designs. The examples called are non exhaustive, and at that place is abundant enquiry on other means of combating fouling organisms through non-toxic coating designs that we have not been able to discuss. For example, protein-degrading enzymes (serine proteases) have been shown to be effective in reducing settlement and adhesion forcefulness of a range of fouling organisms, algal spores, diatoms and barnacle cyprids71, due to dissolution of adhesive72,73. All the same, the challenge for enzyme engineering will be to reach controlled release and stability of enzymes when incorporated into a blanket74,75.
In some cases, the novel coatings we have discussed are existent candidates for practical application after further development work. However, little is known about the machinery(southward) by which these engineered coatings exert their effects. We take shown several examples of correlations between specific types of morphological or chemical surface structuring and biological performance. Withal, correlation does non imply causality and there are real knowledge barriers to understanding how these coatings work. Advances are needed to empathize the underlying biological and molecular mechanisms. For example, at that place is almost no information bachelor for the common macrofouling organisms on what the disquisitional length scale is of the structures involved in surface sensing, nor the molecular ground of their functioning (it is presumed that similar to other jail cell types, spores of seaweeds, barnacle larvae etc possess mechanotransducing proteins and stretch-sensitive ion channels in their surface membranes that can sense and initiate responses to surface structures, simply there are no actual reports of such proteins).
Nosotros accept shown in this review that there is a growing body of evidence that amphiphilic or chemically 'ambiguous' coatings present both polar and non-polar functionalities to a fouling organism and show promising performance against a wide range of fouling organisms; indeed, examples of this blazon of coating, such as Intersleek 900, are bachelor commercially. Even so, relatively little is understood virtually the underlying 'design rules' or the biological basis of performance. Specifically, more understanding of blanket surface properties in the immersed condition, rather than in dry out or in vacuo is needed, as immersion changes the dynamics of cocky-assembly and surface arrangement. For example, in the example of coatings based on amphiphilic PEGylated and fluorinated copolymers, the hypothesis is that these are structured to present a chemically complex surface to fouling organisms. But, is the reduced adhesion observed solely due to the reduced ability of adhesive proteins to adsorb to the PEGylated domains (PEG is well known to be resistant to protein adsorption76) and what are the critical dimensions of the hydrophilic/hydrophobic surface domains? Furthermore, on immersion in the natural marine environment, the surface properties of the coatings may modify further due to surface workout by adsorption of macromolecules and microorganisms77, which will also vary according to season and geographic location. The development of novel, nanorough, fouling-release polysiloxanes containing carbon nanotubes is intriguing, but at present there is no understanding as to the mechanistic basis of this effect; does the nanoroughness cause the cells/larvae to adhere less strongly (that is, a 'biological' ground) or is a 'physical' mechanism more likely whereby nano- and micro-air incursions, such equally those postulated for SHCs36, reduce the interfacial contact betwixt adhesive and surface, thereby reducing adhesion forcefulness? Interdisciplinary studies combining chemistry, biological science and avant-garde physical techniques for interfacial characterization will exist crucial in advancing this field.
The control of biofouling of surfaces in the marine environs is a considerable challenge: the fouling organisms are opportunists and have evolved the capability to attach and proliferate on a range of surfaces in the natural, highly turbulent marine environment. We consider it unlikely that non-biocidal solutions based on coating designs incorporating a unmarried attribute will solve the problem. One way forrad will be to pattern 'multifunctional coatings', incorporating a range of attributes, for case, an appropriate topography combined with a suitable amphiphilic or zwitterionic surface chemistry and environmentally benign compounds that deter settlement or enzymes to target their bioadhesives. Bioinspired 'smart' coatings combining topography with sacrificial or renewable, depression-fouling polymers to shed fouling organisms in a manner analogous to marine mammals78 may emerge equally a practical concept. Fortunately, there is no shortage of innovative ideas! The challenge is to persuade funding agencies that expenditure on enquiry and development will interpret into real benefits in terms of economics and reduced environmental burdens. A recent economic analysisiii shows that a pocket-sized comeback in the condition of the hulls would produce a significant saving. For example, if improved AF technology could produce simply a modest reduction in overall fouling level on ane class of destroyers operated by the US Navy (for example, from 'heavy slime' to 'light slime'), that alone would consequence in a saving of $19 Thou per annum!
Additional information
How to cite this article: Callow, J. A. & Unconversant, One thousand. E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. two:244 doi: ten.1038/ncomms1251 (2011).
References
-
Schultz, M. P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 23, 331–341 (2007). Much-quoted paper reporting the powering penalties of unlike scales of ship hull fouling.
-
Poloczanska, Eastward. Southward. & Butler, A. J. Biofouling and climate change. in Biofouling (eds Durr, S. & Thomason, J.C.) (Wiley-Blackwell, 2010).
-
Schultz, One thousand. P., Bendick, J. A., Holm, E. R. & Hertel, W. 1000. Economic affect of biofouling on a naval surface ship. Biofouling 27, 87–98 (2011)The most up-to-engagement and authoritative analysis of the cost of transport hull biofouling.
-
Jain, A. & Bhosle, N. B. Biochemical composition of the marine workout movie: implications for bacterial adhesion. Biofouling 25, thirteen–19 (2009).
-
Chambers, L. D., Stokes, K. R., Walsh, F. C. & Wood, R. J. K. Mod approaches to marine antifouling coatings. Surf. Coat. Technol. 201, 3642–3652 (2006).
-
Wahl, G. Marine epibiosis i. Fouling and antifouling—some basic aspects. Marine Ecol. Prog. Ser. 58, 175–189 (1989).
-
Yebra, D. Grand., Kiil, S. & Dam-Johansen, Grand. Antifouling engineering-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coating 53, 256–275 (2004).
-
Magin, C. K., Cooper, South. P. & Brennan, A. B. Non-toxic antifouling strategies. Mater. Today 13, 36–44 (2010).
-
Rosenhahn, A., Schilp, Southward., Kreuzer, H. J. & Grunze, Thousand. The function of 'inert' surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 12, 4275–4286 (2010).
-
Callow, M. East., Callow, J. A., Pickett-Heaps, J. D. & Wetherbee, R. Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: quantitative settlement studies and video microscopy. J. Phycol. 33, 938–947 (1997).
-
Roberts, D., Rittschof, D., Holm, East. & Schmidt, A. R. Factors influencing initial larval settlement: temporal, spatial and surface molecular components. J. Exp. Marine Biol. Ecol. 150, 203–221 (1991).
-
Clare, A. S., Rittschof, D., Gerhart, D. J. & Maki, J. S. Molecular approaches to non-toxic antifouling. Invertebr. Reprod. Dev. 22, 67–76 (1992).
-
Rittschof, D. Research on environmentally beneficial antifouling coatings. In Biofouling (eds Durr S. & Thomason J.C.) (Wiley-Blackwell, 2010).
-
Callow, J. A. & Callow, M. E. Biofilms in Antifouling Compounds. In Progress in Molecular and Subcellular Biology: Marine Molecular Biotechnology (eds Fusetani, North. & Clare, A. South.) 141–169 (Springer, 2006).
-
Dobretsov, South., Dahms, H.- U. & Qian, P.- Y. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 22, 43–54 (2006).
-
Huggett, M. J., Williamson, J. E., de Nys, R., Kjelleberg, Southward. & Steinberg, P. D. Larval settlement of the mutual Australian sea urchin Heliocidaris erythrogramma in response to leaner from the surface of coralline algae. Oecologia 149, 604–619 (2006). A comprehensive study of the influence of surface biofilms on the settlement of invertebrate larvae and the start newspaper to show that larvae are able to respond to a bacterial biofilm in the field.
-
Dobretsov, South., Teplitski, 1000. & Paul, V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 25, 413–427 (2009).
-
Huang, S. & Hadfield, M. G. Composition and density of bacterial biofilms make up one's mind larval settlement of the polycheate Hydroides elegans. Mar. Ecol. Prog. Ser. 260, 161–172 (2003).
-
Molino, P. J., Campbell, E. & Wetherbee, R. Development of the initial diatom microfouling layer on antifouling and fouling-release coatings in temperate and tropical Australia. Biofouling 25, 685–694 (2009).
-
Molino, P. J. et al. Development of primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia. Biofouling 25, 149–162 (2009).
-
Aldred, N. & Clare, A. S. Mini-review: the adhesive strategies of cyprids and development of barnacle-resistant marine coatings. Biofouling 24, 351–363 (2008).
-
Smith, A. M. & Callow, J. A. Biological Adhesives (Springer, 2006).
-
Sagert, J., Sun, C. & Waite, J. H. Chemical subtleties of mussel and polychaete holdfasts. In Biological Adhesives (eds Smith A.M. & Callow J.A.) 125–144 (Springer, 2006).
-
Fifty-fifty, Chiliad. A., Wang, J. & Chen, Z. Structural information of mussel adhesive protein Mefp-three acquired at various polymer/Mefp-3 solution interfaces. Langmuir 24, 5795–5801 (2008). An important paper demonstrating the potential of sum frequency generation spectroscopy to report the molecular conformation of marine adhesive proteins on different polymer interfaces.
-
Marechal, J. P., Hellio, C., Sebire, M. & Clare, A. S. Settlement behaviour of marine invertebrate larvae measured by EthoVision 3.0. Biofouling twenty, 211–217 (2004).
-
Aldred, N., Guozhu, 50., Gao, Y., Clare, A. Southward. & Jiang, S. Modulation of barnacle (Balanus amphitrite Darwin) cyprid settlement behavior by sulfobetaine and carboxybetaine methacrylate polymer coatings. Biofouling 26, 673–683 (2010).
-
Zhang, Z., Chao, T., Chen, Due south. F. & Jiang, S. Y. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 22, 10072–10077 (2006).
-
Andersson, O., Ekblad, T., Aldred, N., Clare, A. South. & Liedberg, B. Novel application of imaging surface plasmon resonance for in situ studies of the surface exploration of marine organisms. Biointerphases 4, 65–68 (2009). Novel awarding of iSPR to study the degradation of adhesive 'footprints' of barnacle larvae as they explore surfaces with different physico-chemic properties, in situ and in real time.
-
Kavanagh, C. J., Quinn, R. D. & Swain, Thou. W. Observations of barnacle detachment from silicones using loftier-speed video. J. Adhes. 81, 843–868 (2005).
-
Tribou, M. & Swain, G. The use of proactive in-water training to improve the operation of ship hull antifouling coatings. Biofouling 26, 47–56 (2010).
-
Finnie, A. & Williams, D. N. Paint and coatings technology for the control of marine fouling. In: Biofouling (eds Durr S. & Thomason J.C.) (Wiley-Blackwell, 2010).
-
Brady, R. F. & Singer, I. L. Mechanical factors favoring release from fouling release coatings. Biofouling 15, 73–81 (2000). Seminal paper on the fracture mechanics involved in the release of 'hard-fouling' from elastomeric coatings.
-
Anderson, C., Atlar, M., Callow, M., Candries, M. & Townsin, R. L. The development of foul-release coatings for seagoing vessels. J. Mar. Des. Oper. 84, xi–23 (2003).
-
Bers, A. 5., D'Souza, F., Klijnstra, J. W., Willemsen, P. R. & Wahl, M. Chemical defence in mussels: antifouling effect of crude extracts of the periostracum of the blue mussel Mytilus edulis. Biofouling 22, 251–259 (2006).
-
Ralston, E. & Swain, 1000. Bioinspiration- the solution for biofouling control? Bioinspir. Biomim. 4, 1–9 (2009).
-
Scardino, A. J., Zhang, H., Cookson, D. J., Lamb, R. North. & de Nys, R. The role of nano-roughness in antifouling. Biofouling 25, 749–756 (2009).
-
Genzer, J. & Marmur, A. Biological and synthetic self-cleaning surfaces. MRS Bull. 33, 742–746 (2008).
-
Scardino, A. J. & de Nys, R. Biomimetic models and bioinspired surfaces for fouling command. Biofouling 27, 73–86 (2011).The most recent and extensive review of natural antifouling defences and their potential for biomimetic antifouling solutions.
-
Carman, Yard. L. et al. Engineered antifouling microtopographies—correlating wettability with cell attachment. Biofouling 22, xi–21 (2006).
-
Scardino, A., Guenther, J. & de Nys, R. Zipper point theory revisited: the fouling response to a microtextured matrix. Biofouling 24, 45–53 (2008).
-
Schumacher, J. F. et al. Engineered antifouling microtopographies-result of feature size, geometry and roughness on settlement of zoospores of the green alga Ulva. Biofouling 23, 55–62 (2007). This paper describes the upshot of a systematic approach to understand the role of surface roughness in the attachment of a marine species resulting in a novel quantitative model (the Engineered Roughness Index).
-
Long, C. J. et al. A model that predicts the attachment beliefs of Ulva linza zoospores on surface topography. Biofouling 26, 411–419 (2010).
-
Schumacher, J. F. et al. Engineered nanoforce gradients for inhibition of settlement (attachment) of swimming algal spores. Langmuir 24, 4931–4937 (2007).
-
Schumacher, J. F. et al. Species-specific engineered antifouling topographies: correlations between the settlement of algal zoospores and barnacle cyprids. Biofouling 23, 307–317 (2007).
-
Lin, F.- Y., Chen, W.- Y. & Hearn, M. T. W. Thermodynamic assay of the interaction between proteins and solid surfaces: application to liquid chromatography. J. Mol. Recognit. 15, 55–93 (2002).
-
Krishnan, S. et al. Anti-biofouling properties of rummage-like block copolymer with amphiphilic side-chains. Langmuir. 22, 5075–5086 (2006).
-
Lau, Chiliad. H. A., Blindside, J., Hawker, C. J., Kim, D. H. & Knoll, Westward. Modulation of protein-surface interactions on nanopatterned polymer films. Biomacromolecules 10, 1061–1066 (2009). Why do some heterogeneous, nanopatterned films, such every bit those spontaneously generated from block copolymers, prove reduced adsorption of proteins? This paper systematically explores the influence of feature size on protein adsorption and relates functioning to the density of surface interfaces.
-
Gudipati, C. South., Greenleaf, C. M., Johnson, J. A., Pryoncpan, P. & Wooley, K. L. Hyperbranched fluoropolymer and linear poly(ethyleneglycol) based amphiphilic cross-linked networks every bit efficient antifouling coatings: An insight into the surface compositions, topographies and morphologies. J. Polym. Sci. A Polym. Chem. 42, 6193–6208 (2004).
-
Gudipati, C. S., Finlay, J. A., Callow, Chiliad. Eastward., Callow, J. A. & Wooley, K. Fifty. The anti-fouling and fouling-release performance of unique hyperbranched fluoropolymer (HBFP)-poly(ethylene glycol) (PEG) composite coatings evaluated by protein adsorption and the settlement of zoospores of the green fouling alga Ulva (syn. Enteromorpha). Langmuir 21, 3044–3053 (2005).
-
Krishnan, S. et al. Comparison of the fouling release properties of hydrophobic fluorinated and hydrophilic PEGylated block copolymer surfaces: attachment strength of the diatom Navicula and the dark-green alga Ulva. Biomacromolecules 7, 1449–1462 (2006).
-
Martinelli, E. et al. Nanostructured films of amphiphilic fluorinated block copolymers for fouling release application. Langmuir 24, 13138–13147 (2008).
-
Martinelli, E. et al. Surface engineering science of styrene/PEGylated-fluoroalkyl styrene block copolymer thin films. J. Polym. Sci. A Polym. Chem. 47, 267–284 (2009).
-
Weinman, C. J. et al. ABC triblock surface active block copolymer with grafted ethoxylated fluoroalkyl amphiphilic side chains for marine antifouling/fouling-release applications. Langmuir 25, 12266–12274 (2009).
-
Kristalyn, C. B. et al. Surface structures of an amphiphilic tri-block copolymer in air and in water probed using sum frequency generation vibrational spectroscopy. Langmuir 26, 11337–11343 (2010).
-
Majumdar, P. & Webster, D. C. Grooming of siloxane-urethane coatings having spontaneously formed stable biphasic microtopograpical surfaces. Macromolecules 38, 5857–5859 (2005).
-
Majumdar, P. & Webster, D. C. Influence of solvent composition and degree of reaction on the germination of surface microtopography in a thermoset siloxane–urethane arrangement. Polymer 47, 4172–4181 (2006).
-
Majumdar, P., Stafslien, South., Daniels, J. & Webster, D. C. High throughput combinatorial characterization of thermosetting siloxane–urethane coatings having spontaneously formed microtopographical surfaces. J. Coat. Technol. Res. 4, 131–138 (2007).
-
Sommer, S. et al. A preliminary study on the properties and fouling-release performance of siloxane-polyurethane coatings prepared from poly(dimethylsiloxane) (PDMS) macromers. Biofouling 26, 961–972 (2010).
-
Majumdar, P. et al. Combinatorial materials enquiry applied to the development of new surface coatings 9: an investigation of novel antifouling/fouling-release coatings containing quaternary ammonium salt groups. Biofouling 24, 185–200 (2008).
-
Jiang, S. & Cao, Z. Ultralow-fouling, functionalizable and hydrolysable zwitterionic materials and their derivities for biological applications. Adv. Mater. 22, 920–932 (2010).
-
Zhang, Z. et al. Polysulfobetaine-grafted surfaces every bit environmentally beneficial ultra-depression fouling marine coatings. Langmuir 25, 13516–13521 (2009). First paper to demonstrate the settlement-inhibiting effect of tethered zwitterionic coatings for a marine species.
-
Bennett, S. 1000. et al. The part of surface energy and water wettability in aminoalkyl/fluorocarbon/hydrocarbon-modified xerogel surfaces in the control of marine biofouling. Biofouling 26, 235–246 (2010).
-
Tang, Y. et al. Hybrid xerogel films as novel coatings for antifouling and fouling release. Biofouling 21, 59–71 (2005).
-
McMaster, D. G. et al. Antifouling character of 'agile' hybrid xerogel coatings with sequestered catalysts for the activation of hydrogen peroxide. Biofouling 25, 21–33 (2009).
-
Finlay, J. A. et al. Barnacle settlement and the adhesion of protein and diatom microfouling to xerogel films with varying surface energy and water wettability. Biofouling 26, 657–666 (2010).
-
Beigbeder, A. et al. Preparation and characterisation of silicone-based coatings filled with carbon nanotubes and natural sepiolite, and their awarding as marine fouling-release coatings. Biofouling 24, 291–302 (2008).
-
Begbeider, A. et al. CH-p interactions as the driving forcefulness for silicone-based nanocomposites with exceptional properties. Adv. Mater. 20, 1003–1007 (2008).
-
Beigbeder, A. et al. On the effect of carbon nanotubes on the wettability and surface morphology of hydrosilylation-curing silicone coatings. J. Nanostruct. Polym. Nanocomposites 5, 37–43 (2009).
-
Marmur, A. Adhesion and wetting in an aqueous environment: theoretical assessment of sensitivity to the solid surface energy. Langmuir 20, 1317–1320 (2004).
-
Thomas, Yard. V. & Brooks, S. The environmental fate and effects of antifouling pigment biocides. Biofouling 26, 73–88 (2010).
-
Pettitt, M., Henry, South., Callow, M., Callow, J. & Clare, A. Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling 20, 299–311 (2004).
-
Phang, I. Y. et al. Atomic force microscopy of the morphology and mechanical behaviour of barnacle cyprid footprint proteins at the nanoscale. J. Roy. Soc. Interface 24, 97–107 (2008). First application of AFM to study the mechanical properties of the temporary footprint agglutinative deposited by barnacle larvae every bit they explore surfaces.
-
Aldred, N., Phang, I. Y., Conlan, S. L., Clare, A. S. & Vansco, M. J. The effects of a serine protease, Alcalase® on the adhesives of barnacle cyprids (Balanus amphitrite). Biofouling 24, 97–107 (2008).
-
Tasso, M. et al. Antifouling potential of Subtilisin A immobilized onto maleic anhydride copolymer thin films. Biofouling 25, 505–516 (2009).
-
Tasso, Chiliad., Cordeiro, A. L., Salchert, Yard. & Werner, C. Covalent immobilization of Subtilisin A onto Thin Films of Maleic Anhydride Copolymers. Macromol. Biosci. 9, 922–929 (2009).
-
Jeon, S. I., Lee, J. H., Andrade, J. D. & de Gennes, P. M. Protein-surface interactions in the presence of polythylene oxide: I. Simplified theory. J. Colloid Interface Sci. 142, 149–158 (1991).
-
Huggett, Thou. J., Nedved, B. T. & Hadfield, M. G. Furnishings of initial surface wettability on biofilm formation and subsequent settlement of Hydroides elegans. Biofouling 25, 387–399 (2009).
-
Ganguli, R., Mehrotra, 5. & Dunn, B. Bioinspired living skins for fouling mitigation. Smart Mater. Struct. 18, i–8 (2009).
Acknowledgements
The authors acknowledge back up from the AMBIO projection (NMP-CT-2005-011827) funded by the European Commission'due south 6th Framework Programme, and support from the US Office of Naval Enquiry (N00014-08-1-0010). Dr Nick Aldred and Professor Tony Clare (University of Newcastle) are thanked for comments on Figures 3 and 4.
Author information
Affiliations
Respective author
Ideals declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
Virtually this commodity
Cite this article
Unconversant, J., Unconversant, G. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat Commun 2, 244 (2011). https://doi.org/10.1038/ncomms1251
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/ncomms1251
Further reading
Comments
Past submitting a comment you hold to abide by our Terms and Community Guidelines. If you notice something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
crosswhitepronow1963.blogspot.com
Source: https://www.nature.com/articles/ncomms1251
0 Response to "Organisms Colonization Growth and Evolution in a Friendly Environment Review"
إرسال تعليق